Explain the Different Types of Drug Formulations
Buffer Capacitythe strength of a buffer system. Asava Arista la 15.

Introduction Classification And Definitions Of Dosage Forms Pharmaceutical Guidelines
Unsuitable for those who are experiencing severe vomiting or have difficultyswallowingAlso.
. Pharmaceutical formulation is the multistep process where the active drug is mixed with all other components. Brief overview of the different forms of drug formulation development 4. Nongranular suspensions can be administered via enteral feeding tubes but may require further dilution owing to.
This class broadly encompasses two types of formulation tablets and capsules. Some examples of CRTs are transdermal and transmucosal controlled-release delivery systems ml6 nasal and buccal aerosol sprays drug-impregnated lozenges encapsulated cells oral soft gels iontophoretic devices to administer drugs through skin and a variety of programmable implanted drug-delivery devices. There are different types of tablets but all look similar except in terms of shape size and color.
Oral drugs are normally taken as tablets or capsules. A pharmaceutical formulation is composed of several formulation factors and process variables. Emulsionin colloid chemistry a suspension of one liquid oil in another aqueous.
Like capsules tablets and pills etc. Understanding the chemical basis of drug stability and degradation. The amount of acid or base that can be added before the solution pH shifts.
Liquid formulations have been widely used in pharmaceutics due to their high dosing flexibility ease of swallowing and quick onset of action. The drug active substance itself needs to be soluble in aqueous solution at a controlled rate. Especially used to control drug release in modified-release formulations prolonged-release or controlled-release tablet.
Others include powders granules etc. We would like to show you a description here but the site wont allow us. Often generic drug names will be used by healthcare providers and the general public without the salt ending just out of convenience.
The first is called as ligand-based drug design and the second structure-based drug design. But in fact tablets are designed after careful thought by pharmacists. Typically they are categorized as monophasic and biphasic formulations wherein within these two broad categories lies a wide range of dosage forms Fig.
The official name is tramadol hydrochloride but commonly it is often. This means that other manufacturers that may wish to develop alternative formulations or generic versions of the drug will not be able to have their products approved during the. Small tablet containing excipients Lozenges.
Slow-release medications may extend the duration of the effect. Describe the mechanisms of drug degradation and provide examples of each 5. Summarize approaches employed to stabilize drugs in pharmaceutical dosage forms 7.
Classifications for Pharmaceutical Formulation and Drug Design Drug design There are two major types or classifications of drug design. Different than patents Marketing Exclusivity is a key drug development incentive. Astles and Janice Davies discuss the chemical basis for the stability of certain functional groups within drug molecules against hydrolytic degradation and consider the methods used to maximise their stability on storage.
The main challenge of all the different parenteral dosage forms is to achieve a good compatibility of the drug substances with the excipients no formation of new impurities either by degradation of the drug substance or formation of new chemical entity between the drug substance and the excipients as well as the compatibility of. It has to be a particular configurationcapsule shell for example and distributed into a particular dose. Types and Forms of Ayuvedic formulations Solid dosage forms.
Tier 2 40 copay Mainly higher-cost generic drugs and the low-cost brand name drugs Tier 3- 60 copay For brand name drugs where there is no generic alternative Tier 4 100 copay Highest-cost drugs or specialty drugs such as chemotherapy The above copays may slightly differ from your plan. Solid dosage containing medicaments with suitable suppository base that inserted in to the body. Used for low solubility tablets to improve wetting and deaggregation of drug particles to get a rapid and improved dissolution.
The dosage form is the pharmaceutical drug product as marketed for use with a specific mixture of active ingredients and inactive components. Others include powders granules etc. Challenges in Formulations.
Understand the types timelines of different options for your program. Different Forms of Drug Formulation 1. Medications are formulated to avoid stomach acids and digestive enzymes.
Drugs enclosed with wafer sheet of rice Pills. Avaleha Ghrita Liquid dosage forms. Ligand-based drug design- In this branch or type of.
In this science article Timothy J. Pros Cons of Different Routes of Drug Administration 1. Quantitative model-based pharmaceutical formulation involves establishing mathematical relations between the formulation variables and the resulting responses and optimizing the formulation conditions.
A suspension formulation is usually developed when the drug is insoluble or if for reasons of palatability the drug is formulated into coated microgranules. Gutika Chruna Semi solid forms. Describe the fi ve types of drug instability of concern to the practicing pharmacist 6.
Different Forms of Drug Formulation 2. So for example while the narcotic-like painkiller tramadol and tramadol hydrochloride may sound like two different drugs they are actually the same drug. The drug form varies by the route of administration.
Pharmaceutical Formulation Pharmaceutical formulation is the process of combining various chemical substances with. Drug enclosed with gelatin capsule Cachets. Solid preparations containing sugar and gum used to medicate mouth and throat Suppositories.
They are designed to meet the patients requirements disease state medicine content and other factors. Drug substance for possible inclusion into a dosage form 4. Buffer Systema weak acid and its conjugate salt that prevents the pH from shifting in a solution.

The Role Of Nanotechnology In Drug Discovery Drug Discovery World Ddw

Modified Release Alternative Strategies For Development Of Modified Release Dosage Forms
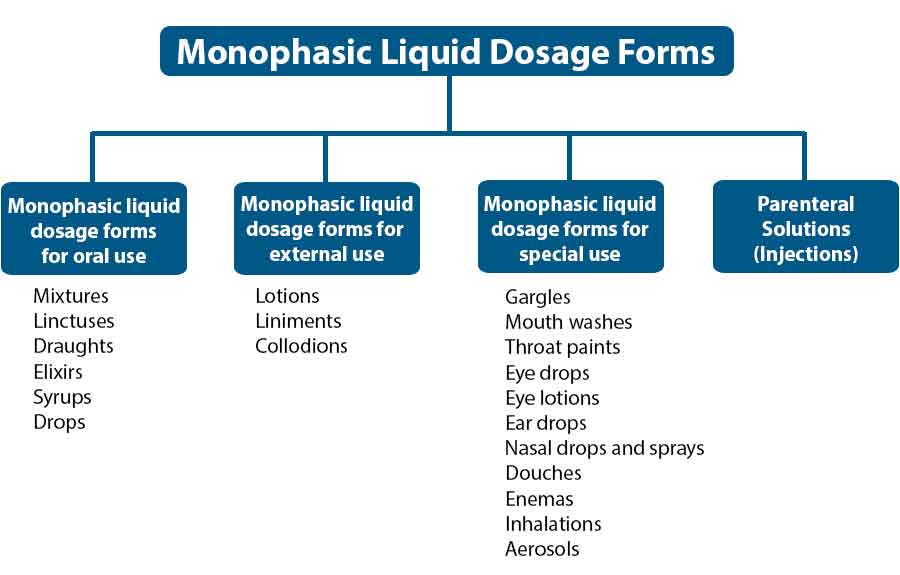
Understanding Pharmaceutical Liquid Dosage Forms
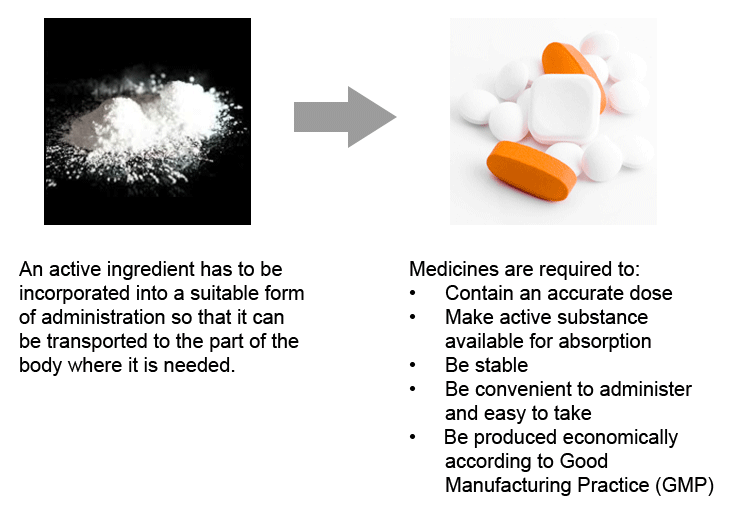
Galenic Formulation How Medicines Are Formulated Eupati Toolbox

The Central Role Of Excipients In Drug Formulation European Pharmaceutical Review

Challenges And Opportunities In Oral Formulation Development American Pharmaceutical Review The Review Of American Pharmaceutical Business Technology
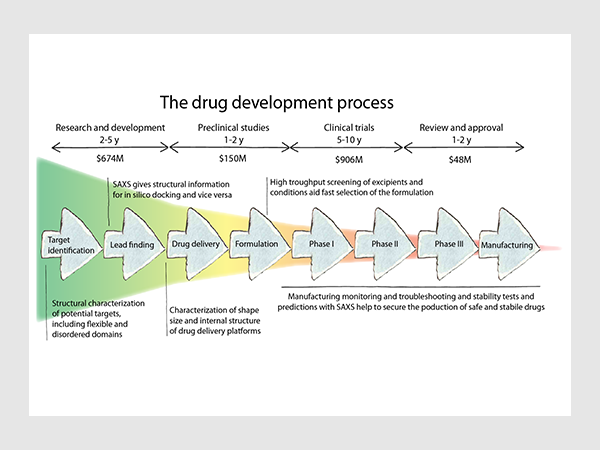
Saxs Protein Saxs For Drug Development And Formulations

Liquid Formulation An Overview Sciencedirect Topics

Drug Delivery System An Overview Sciencedirect Topics
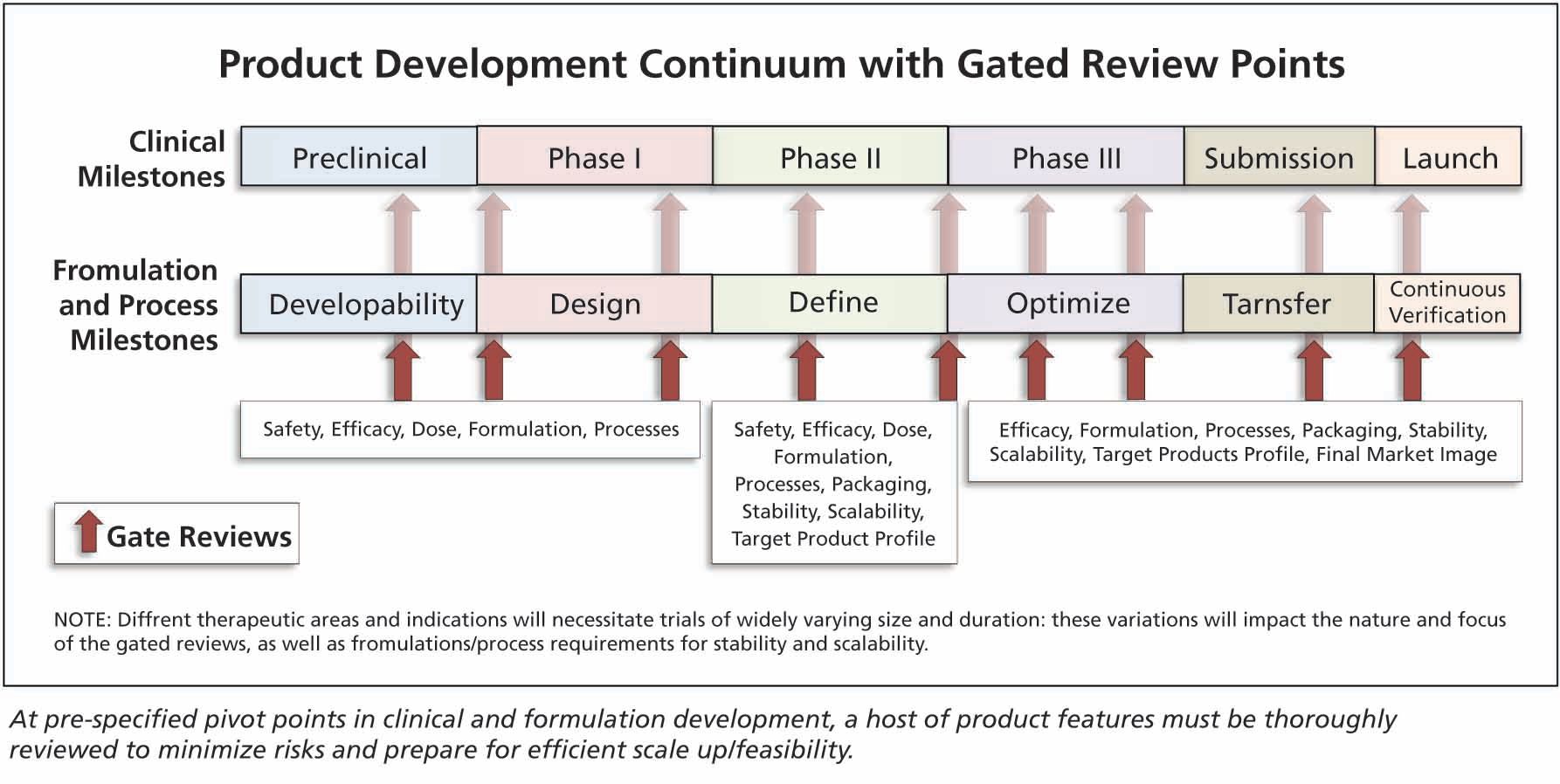
Phase Appropriate Formulation And Process Design

Dosage Form Short Notes Docsity

Introduction Classification And Definitions Of Dosage Forms Pharmaceutical Guidelines

Controlled Release Formulation An Overview Sciencedirect Topics
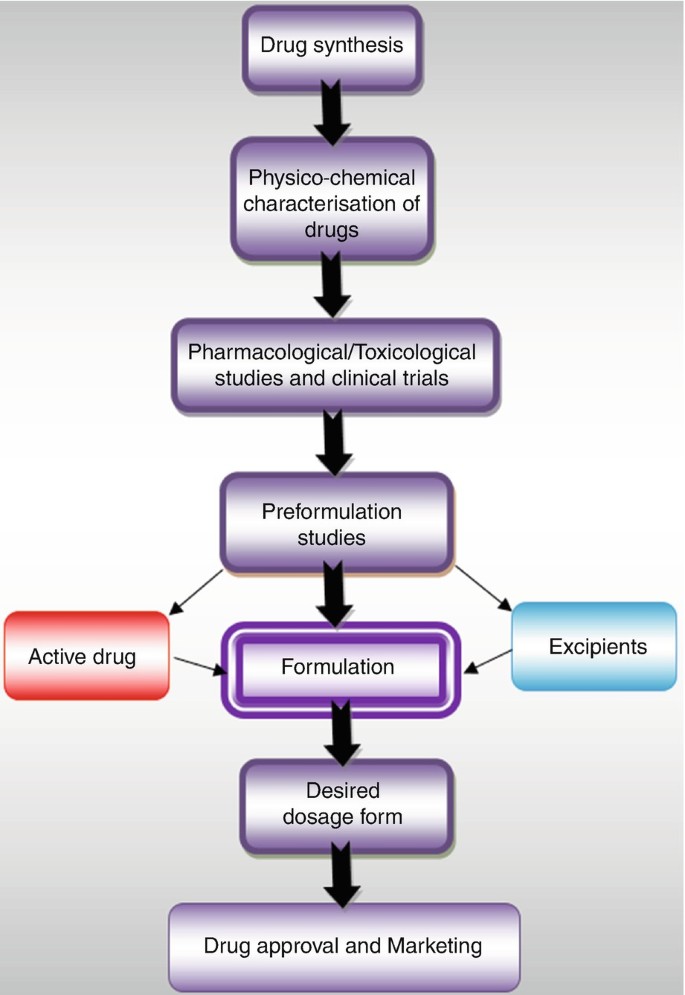
Drug Formulations Springerlink
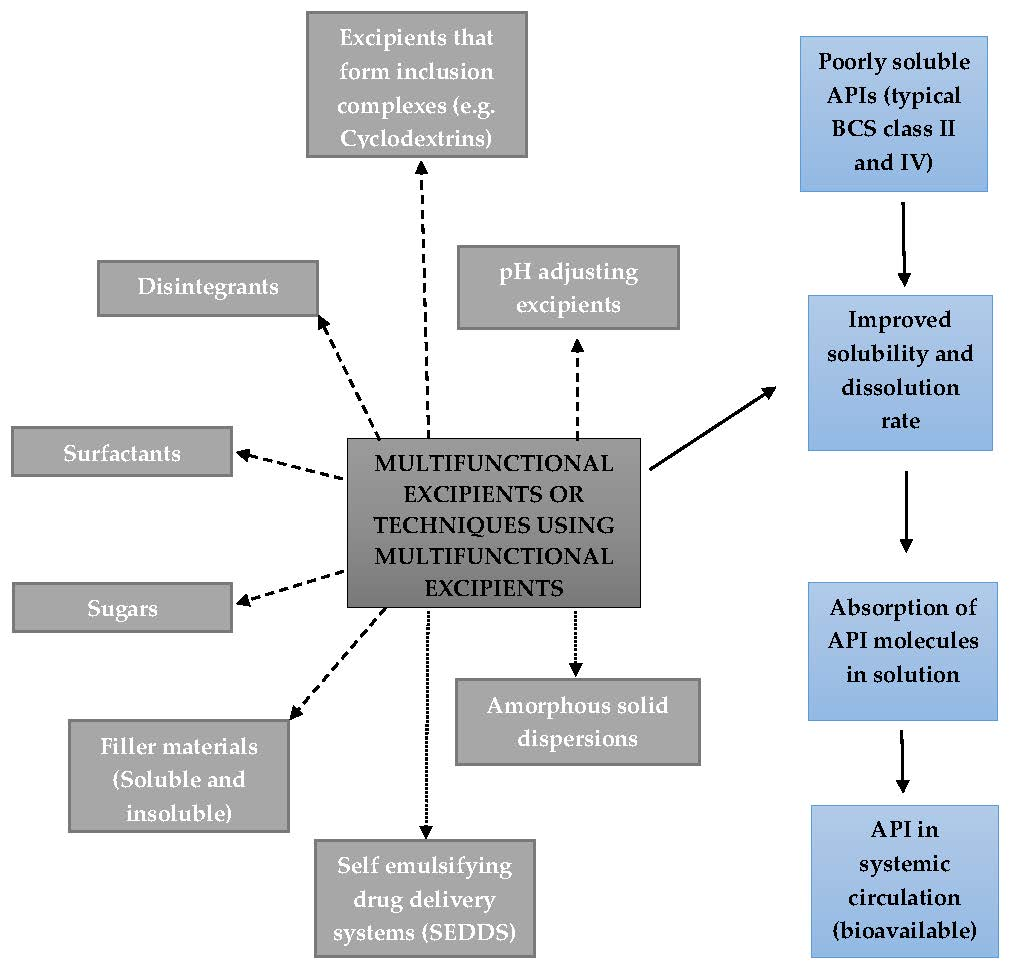
Pharmaceutics Free Full Text The Role Of Functional Excipients In Solid Oral Dosage Forms To Overcome Poor Drug Dissolution And Bioavailability Html

Multiparticulate Formulations Using Multiparticulate Technology To Develop Pediatric Drug Products
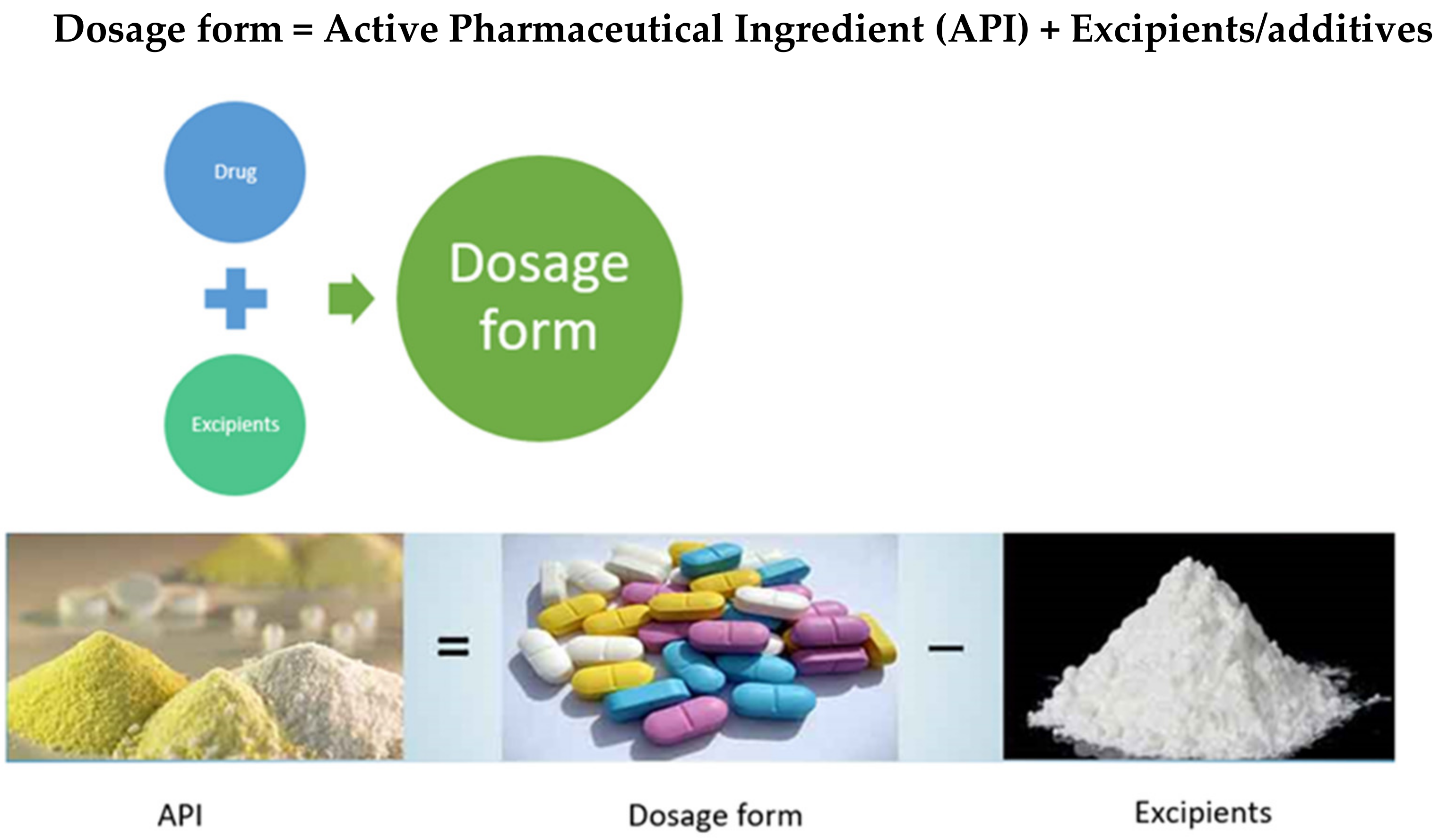
Molecules Free Full Text Controlled Drug Delivery Systems Current Status And Future Directions Html


Comments
Post a Comment